 Mr. Shoucheng Du, Prof. Julia Valla, and Prof. George Bollas are making exciting progress in developing the process of biomass catalytic pyrolysis. Their recent achievements are published in Green Chemistry (link to article), and were presented at the 2013 Spring Meeting of the American Chemical Society (link to presentation).
Mr. Shoucheng Du, Prof. Julia Valla, and Prof. George Bollas are making exciting progress in developing the process of biomass catalytic pyrolysis. Their recent achievements are published in Green Chemistry (link to article), and were presented at the 2013 Spring Meeting of the American Chemical Society (link to presentation).
Biomass pyrolysis is the thermal decomposition of solid biomass into a liquid, which after additional processing, can be employed in the manufacture of chemicals, fuels, and other products normally made from petroleum.
According to Mr. Du, a Chemical & Biomolecular Engineering (CBE) graduate student, biomass pyrolysis is one of the process options most likely to solve the challenge of renewable fuels. “We let nature and photosynthesis develop biomass, such as plants and trees, from carbon dioxide in the air,” Mr. Du says, “then our work focuses on upgrading the value of that natural product, lignocellulosic biomass, into liquid bio-oil, which can then be upgraded by a catalyst into liquid products of more value to society.”
“The challenge,” says Prof. Bollas, “is that the byproducts of pyrolysis, coke and char, deactivate the catalyst by coating the surface. Hence, the most important objective is to first identify the exact amounts of coke (a catalytic product) and char (the thermal byproduct of pyrolysis) that lead to deactivation, which will further our understanding of the reaction mechanisms.”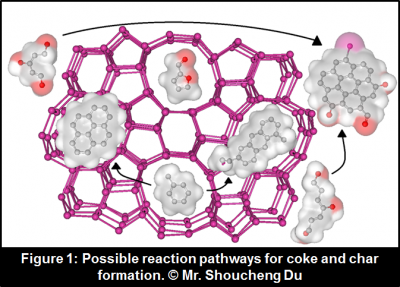
Prof. Valla is studying the related issue of tar, a thick viscous form of the liquid bio-oil. “When we focus on biomass tar, the challenge is even greater. Coke dominates the product distribution and it would be invaluable to understand how it is formed,” she comments.
By studying the reactions likely to lead to coke and char, and the properties of the catalyst used (Figure 1), Prof. Bollas’ group was able to identify hemicellulose as a dominant coke precursor, separate the pathways that lead to the formation of coke and char, and propose possible reactions to minimize the deactivation of the catalyst.
“Now the challenge is to connect these findings to the production of the useful liquid product,” Prof. Bollas says. “We believe the same precursors produce the most desired and most undesired products.” In the future, Prof. Bollas and his team will continue to study these reactions further, to perhaps determine a method to control their negative side effects.